Model of the crystal structure
-
 Phase field model -- some of both crystals everywhere
Phase field model -- some of both crystals everywhere
- Mass fraction of round crystal type at every point
as u(t)
- Square crystal type at every point is 1-u(t)
-
 Thermodynamics of mixed
phases at mild temperatures
Thermodynamics of mixed
phases at mild temperatures
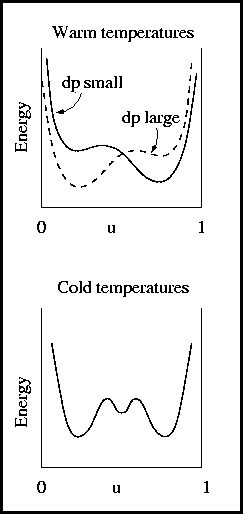
- Two wells in the energy potential -> two phases
- Energy barrier between two phases -> inhibits change
- Pressure gradient determines the lower energy state
- Squares prefered if dp is large
- Rounds prefered if dp is small
-
 Thermodynamics of mixed
phases at cold temperatures
Thermodynamics of mixed
phases at cold temperatures
- Three wells in the energy potential
- Allows for persistant equal mixture of phases
- Inhibits square or round formation
-
 Simplified model
u' = - dE(u,dp,T) / du
Simplified model
u' = - dE(u,dp,T) / du
- Decrease energy as rapidly as possible
- Changes mass fraction of crystal types


Phase field model -- some of both crystals everywhere
Thermodynamics of mixed phases at mild temperatures
Thermodynamics of mixed phases at cold temperatures
Simplified model